| By Alpha|By Interest|Emeritus | ||
2022 MBNI Building |
(734) 763-3725
|
 |
Current Research Interests:Emotional regulation of complex behaviors such as reinforcement, feeding, and stress are central to proper functioning of both animals and humans. This laboratory focuses on CNS circuits and cellular systems that participate and regulate these states in the brains of individuals with severe mental illness. Using a variety of molecular, anatomical, behavioral and pharmacological approaches, it is possible to focus on key circuits and molecules of interests. These projects cover a wide range of topics: 1. Depression, bipolar disease and schizophrenia are all studied in postmortem human brain using microarrays across several regions of CNS. Families of genes have been found to be disregulated in these three major illnesses and are being further evaluated in animal studies. Most recently we and other members of the Pritzker Neuropsychiatric Disease Research Consortium have shown significant changes in Fibroblast Growth Factor, Mitochondria and immune gene families in major depression, bipolar disease, and schizophrenia respectively. In the near future broad-based genetic studies will be initiated in large sized patient sets.
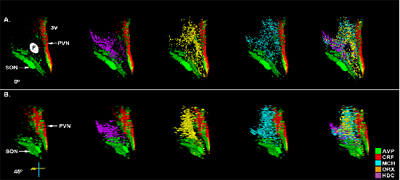
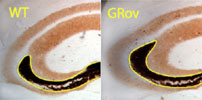
2. Stress and substance abuse responsive systems have been mapped in rodent CNS using in situ hybridization of mRNA and intronic RNA for a large number of candidate molecules. By combining in situ regulatory studies with track tracing and other anatomical tools we have been able to describe how the brain responds to stress. Mixing these basic strategies with genetically altered mice has provided rich perspectives on the roles of the glucocorticoid receptor in mice. Similar analyses in high vs. low responder rats has been very powerful in revealing propensity to use drugs of abuse, for understanding some aspects of maternal behavior and for risk taking behavior. The regulation of important neural circuits is clearly altered in such animals.
Selected Publications:Akil, H., Evans, S.J., Turner, C.A., Perez, J., Myers, R.M., Bunney, W.M., Jones, E.G., Watson, S.J., and Pritzker Consortium: The fibroblast growth factor family and mood disorders. Novartis Foundation Symposium 289, 94-96, 2008. Cecchi, M., Capriles, N., Watson, S.J., Akil, H.: Differential responses to morphine-induced analgesia in the tail-flick test. Behavioural Brain Research 194:146-151, 2008. Flagel, S.B., Watson, S.J., Akil, H., Robinson, T.: Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav. Brain Res., 186:48-56, 2008. Turner, C.A., Flagel, S.B., Clinton, S.M., Akil, H. and Watson, S.J.: Cocaine interacts with the novelty-seeking trait to alter FGFR1 gene expression in the rat. Neuroscience Letters 446, 105-107, 2008. Bernard, R., Kerman, I.A., Meng, F., Evans, S.J., Amrein, I., Jones, E.G., Bunney, W.E., Barchas, J.D., Myers, R.M., Schatzberg, A.F., Akil, H., Watson, S.J., Thompson, R.C.: Gene expression profiling of neurochemically-defined regions of the postmortem human brain by an in situ hybridization-guided laser capture microdissection. Journal of Neuroscience Methods 178: 46-54, 2009. Evans, S.J., Akil, H., Watson, S.J.: Analyzing gene expression in depression. Amer. Journal of Psychiatry 166:9: September 2009. Garcia-Fuster, M.J., Clinton, S.M., Watson, S.J., Akil, H.: Effect of cocaine on Fas-associated protein with death domain in the rat brain: individual differences in a model of differential vulnerability to drug abuse. Neuropsychopharmacology 34:1123-1134, 2009. Rollins, B., Martin, M., Sequeira, P.A., Moon, E., Morgan, L., Watson, S.J., Schatzberg, A., Akil, H., Myers, R., Jones, E., Wallace, D., Bunney, W., Vawter, M.: Mitochondrial variants in schizophrenia, bipolar disorder, and major depressive disorder. PLoS One, Vol. 4, Issue 3, e4913, March 2009. Perez, J.A., Clinton, S.M., Turner, C.A., Watson, S.J., Akil, H.: A new role for FGF2 as an endogenous inhibitor of anxiety, J. Neuroscience 29(19):6379-6387, 2009. Martin, M.V., Rollins, B., Sequeira, P.A., Mesen, A., Byerley, W., Stein, R., Moon, E.A., Akil, H., Jones, E.G., Watson, S.J., Barchas, J., DeLisi, L.E., Myers, R.M., Schatzberg, A., Bunney, W.E., Vawter, M.P.: Exon expression in lymphoblastoid cell lines from subjects with schizophrenia before and after glucose deprivation. BMC Med Genomics. 2:62, 2009. Garcia-Fuster, M.J., Perez, J.A., Clinton, S.M., Watson, S.J., Akil, H.: Impact of cocaine on adult hippocampal neurogenesis in an animal model of differential propensity to drug abuse. European Journal of Neuroscience (1):79-89, 2010. Bernard, R., Kerman, I.A., Thompson, R.C., Jones, E.G., Bunney, W.E., Barchas, J.D., Schatzberg, A.F., Myers, R.M., Akil, H., Watson, S.J.: Altered expression of glutamate signaling, growth factor and glia genes in the locus coeruleus of patients with major depression. Molecular Psychiatry, 1-13, 2010. Krolewski, DM, Medina, A., Kerman, IA, Bernard, R, Burke, S, Thompson, R, Bunney, WE, Schatzberg, AF, Myers, RM, Akil, H, Jones, EG, Watson, SJ: Expression patterns of hypothalamic pituitary adrenal axis-related genes in the human hypothalamus. Submitted to the Journal of Comparative Neurology, March 2010.
|
||

 |
UM Gateway | UMMS | UMHS |
| Copyright © 2005 The Regents of the University of Michigan | |