| By Alpha|By Interest|Emeritus | ||||
5045 BSRB |
734-936-2057 |
 |
||
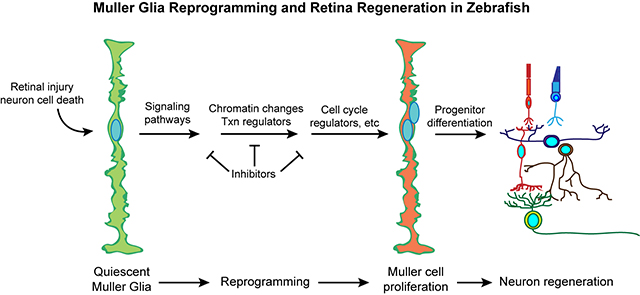
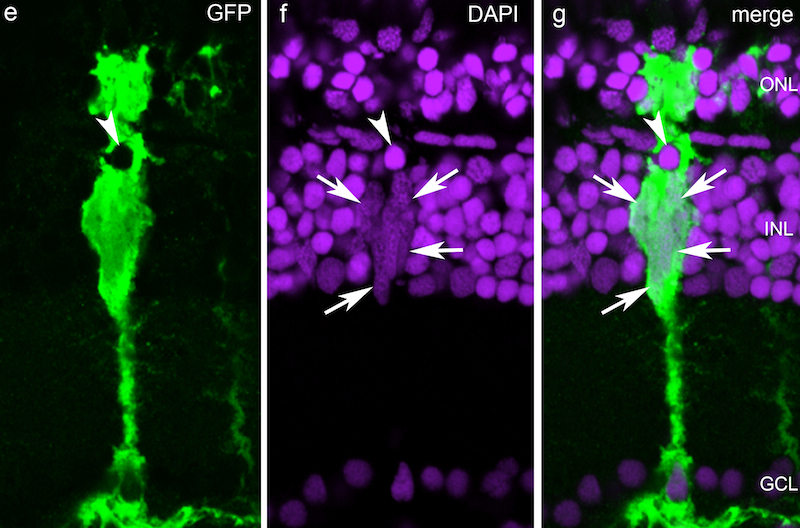
Current Research Interests:Our lab is interested in stimulating regeneration in the central nervous system (CNS). Death of CNS neurons underlies many neurodegenerative diseases and injuries that result in functional deficits. The retina is part of the CNS and provides an ideal system for studying CNS regeneration because of its relatively simple structure and accessible to experimental manipulation. Neurodegenerative diseases of the retina include macular degeneration and glaucoma which cause blindness and are among the top 10 disabilities affecting people. Unfortunately, mammals cannot regenerate their CNS. However, hope comes from teleost fish, like zebrafish, that have remarkable regenerative abilities and can regenerate neurons in the retina and brain. Because the zebrafish and mammalian retina share structure and function, we suspect that mechanisms driving retina and optic nerve regeneration in zebrafish will suggest regenerative strategies that can be applied to mammals. Furthermore, mechanisms underlying retina and optic nerve regeneration may inform us of strategies for stimulating regeneration throughout the CNS. In the retina of fish and mammals there is only one major glial cell type referred to as a Muller glial cell. These are radial cells whose processes span the retina and interact with neighboring neurons. Normally Muller cells contribute to retina structure and homeostasis. However, in zebrafish, Muller glia respond to retinal injury by undergoing a reprogramming event that endows them with properties of a retinal stem cell. These reprogrammed glial cells divide to produce a retinal progenitor cell that is capable of regenerating all major retinal neuron types. Using a variety of molecular, genetic, biochemical, and cell biological approaches, we have identified and characterized many of the genomic events and signaling pathways that underlie Muller glial cell reprogramming and retina regeneration. This information is being used to suggest strategies for stimulating retina and CNS regeneration in mammals.
Honors and Awards:
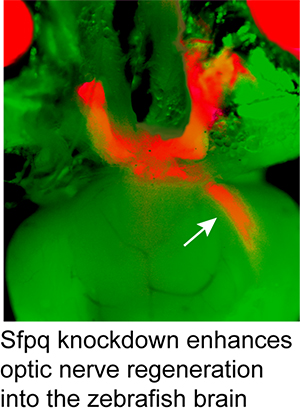
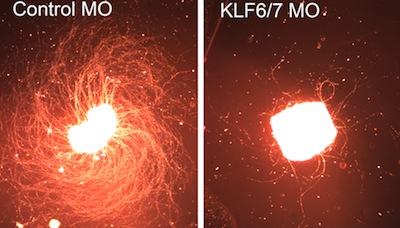
1994 University of Michigan, Research Scientist Recognition Award Selected Publications:Notch Suppression Collaborates with Ascl1 and Lin28 to Unleash a Regenerative Response in Fish Retina, But Not in Mice. Opposing Actions of Fgf8a on Notch Signaling Distinguish Two Muller Glial Cell Populations that Contribute to Retina Growth and Regeneration. Retina regeneration in zebrafish. Zebrafish Muller glia-derived progenitors are multipotent, exhibit proliferative biases and regenerate excess neurons. Leptin and IL-6 Family Cytokines Synergize to Stimulate Müller Glia Reprogramming and Retina Regeneration Retinal Injury, Growth Factors, and Cytokines Converge on β-Catenin and pStat3 Signaling to Stimulate Retina Regeneration Zinc-binding domain dependent, deaminase-independent actions of apolipoprotein B mRNA editing enzyme, catalytic polypeptide 2 (Apobec2) mediate its effect on zebrafish retina regeneration. Excitotoxic brain injury in adult zebrafish stimulates neurogenesis and long-distance neuronal integration. Regeneration, morphogenesis and self-organization. Müller glial cell reprogramming and retina regeneration. Jak/Stat signaling stimulates zebrafish optic nerve regeneration and overcomes the inhibitory actions of Socs3 and Sfpq. Analysis of DNA methylation reveals a partial reprogramming of the Müller glia genome during retina regeneration. Insm1a-mediated gene repression is essential for the formation and differentiation of Müller glia-derived progenitors in the injured retina. HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration. Injury-dependent Müller glia and ganglion cell reprogramming during tissue regeneration requires Apobec2a and Apobec2b. Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration. Ascl1a regulates Müller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Conditional gene expression and lineage tracing of tuba1a expressing cells during zebrafish development and retina regeneration Tuba1a gene expression is regulated by KLF6/7 and is necessary for CNS development and regeneration in zebrafish. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration. A reporter-assisted mutagenesis screen using alpha 1-tubulin-GFP transgenic zebrafish uncovers missteps during neuronal development and axonogenesis. A role for alpha1 tubulin-expressing Müller glia in regeneration of the injured zebrafish retina. Complete List of Published Work in My Bibliography |
||||

 |
UM Gateway | UMMS | UMHS |
| Copyright © 2005 The Regents of the University of Michigan | |